Molecular identification
Our fungal isolate showed a high initial screening task and obvious growth; thus, it was also utilized for biosynthesizing our SeNPs and examining the antibacterial, antifungal, antioxidant and cancer-fighting properties60. Our isolated fungal species created white mycelia throughout its vegetative stage, and it developed green sporulation over the sporulation process40. The fungal isolate’s 28S rRNA sequences were matched with genetically similar strains from GenBank. Under the accession number OR059437, the sequence was entered into the database of GenBank. A phylogenetic tree (Fig. 1) was established via the method of neighbor-joining in the MEGA11 software package to show the degree of similarity between the acquired sequence and the reference sequences in the genomic database.

Neighbor-joining phylogenetic tree of P. tardochrysogenum OR059437and related fungi.
SeNPs synthesis by P. tardochrysogenum OR059437
Brick-red replaced the previous yellow hue in the culture medium after 30min of incubation when treating the culture filtrate with 1 mM Na2SeO4 (Fig. 2A).
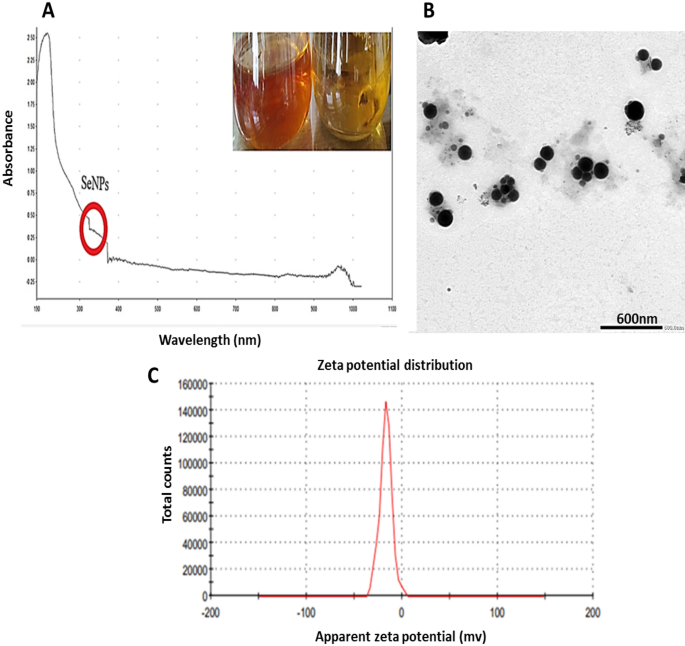
(A) UV- Visible spectrum of myco-synthesized Se-NPs using P. tardochrysogenum OR059437, (the red circle indicated the SeNPs formation range), (B): TEM image of myco-synthesized Se-NPs of scale 600 nm, (C): Zeta potential distribution of myco-synthesized Se-NPs.
The metabolites secreted extracellularly swiftly converted ions of selenite into elemental Se (Se0) form since culture media after incubation exhibited a red-brick appearance61.
Characterization of Myco-synthesized SeNPs
Developing selenium NPs in the culture filtrate was observed by UV–visible spectrophotometry, which showed apronounced shoulder at about 350 nm, a SeNPs characteristic (Fig. 2A). This outcome is consistent with that of Yedurkar et al.62. The UV absorbance peak of the phycosynthesized Polycladia myrica SeNPs was 350. Experiments made by Morad, et al.61 illustrated a surface plasmon resonance (SPR) peak at 521 nm, that is a property of selenium NPs, was highly and broadly visible as a result of the formation of SeNPs in the filtrate of Penicillium chrysogenum Z945518.
A colloidal solution of myco-produced SeNPs was examined using TEM as shown in (Fig. 2B,C); indicating the production of poly-dispersed spherical SeNPs with diameters from 60.21 to 104.41 nm. These investigations are reliable with those made by El-Shanshoury et al.63, when examined Bacillus subtilis to create selenium nanoparticles and found that the resulting particles were polydispersed and spherical. The experiments made by Morad, et al.61, were acceptable with our findings as this previous study demonstrated SeNPs produced from penicillium chrysogenum Z945518, with sizes between 44 to 78 nm.
Depending on the zeta potential analysis of the charge on the particle’s surface, the stability of selenium NPs was assessed, which resulted in a mean zeta potential of − 17 mV (Fig. 2D). Our measurements was acceptable based on the research of Morad et al.61, who claimed that the SeNPs formed by penicillium chrysogenum Z945518 observed a mean zeta potential of − 32.4 mV. Furthermore, according to Dumore and Mukhopadhyay64, it was found that the negative zeta potential is helpful for anti-oxidation and the use of nanoparticles in cancer (A549) cells. These observations may be attributed to the surface of SeNPs revealing the -ve charge of the OH and COO groups. The higher stability of these SeNPs is shown by a bigger zeta potential magnitude.
Through EDX analysis; selenium elemental composition was shown in (Fig. 3A,B); PeSeNPs had an atomic and mass percentage: 0.16% ± 0.01 and 0.88% ± 0.06, respectively. This proves SeNPs were prepared. Some other noticed EDX peaks were recorded including C, O, Na, and Cl, with mass percentages of 40.09 ± 0.17, 57.81 ± 0.37, 0.71 ± 0.04, and 0.51 ± 0.03, atom percentages of 47.64 ± 0.20, 51.56 ± 0.33, 0.44 ± 0.03 and 0.20 ± 0.01, respectively.
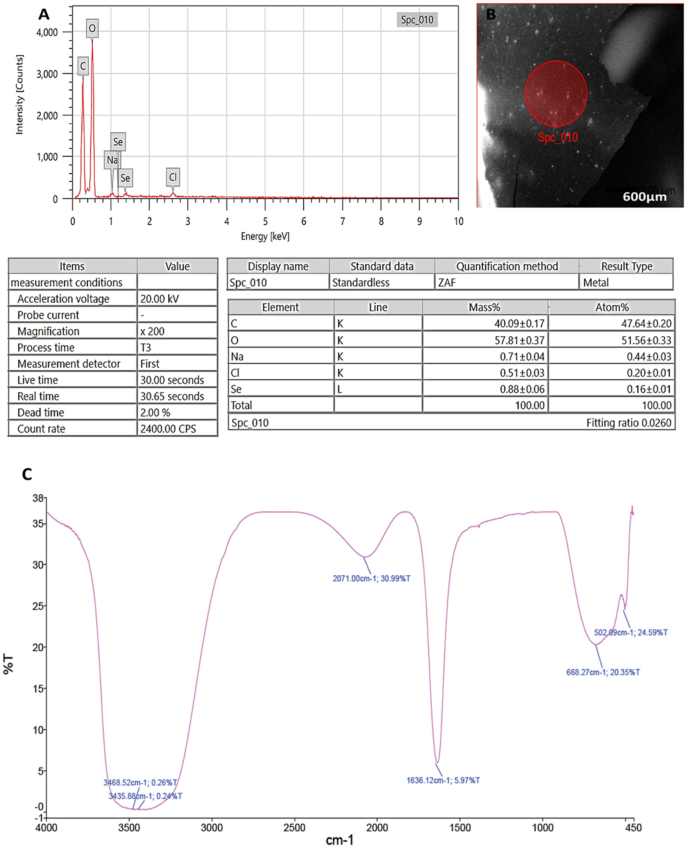
(A): EDX analysis of PeSeNPS, (B): Field of EDX analysis of PeSeNPs, (C): FT-IR spectrum of PeSeNPs.
FTIR analyses were utilized for identifying the appearance of several functional groups in metabolites that are in charge of SeNP synthesized from fungi, capping, and stability. The PeSeNPs’ FT-IR spectra (Fig. 3C), exhibited absorption peaks at 3468.52, 3435.88, 2071.00, 1636.12, 668.27, and 502.09 cm−1 in the area of 450 to 4000 cm−1. The high absorption bands suggest the presence of alcohols at 3468.52–3435.88 cm−1 in the PeSeNPs illustrating the O–H stretching65,66. 2071.00 cm−1 band allocated to C=C stretching in alkenes, C=C phenyl compounds stretching, and C=O of aromatic amide I stretching (proteins and peptides) are represented by the band at 1636.12 cm−1 of the spectra, as stated by Demir et al.67, band at 668.27 cm−1 and 502.09 cm−1 bands were related to C–Cl stretching group.
In the same line Barabadi, et al.68, found that; Penicillium chrysogenum PTCC 5031; FT-IR spectrum SeNPs showed absorption peaks between 450 and 4000 cm−1 at 1088.38, 1412.66, 1632.57, and 3440.33 cm−1. The C–N stretching vibration of the amine is represented by the band at 1088.38 cm−1. Primary amides’ N–H stretching vibration is represented by the band at 1412.66 cm−1. Additionally, C–C and O–H stretching were attributed to peaks at 1632.57 and 3440.33 cm−1, respectively. FT-IR results demonstrated the existence of functional groups on the surface of SeNPs. The observed functional groups are a result of the conjugated biomolecules acting as stabilizing and reducing agents on selenium nanoparticle’s surface.
Antioxidant activity of myco-synthesized PeSeNPs
However, DPPH radical scavenging activity of PeSeNPs (Fig. 4) reached higher than 90% at 1000 µg/ml of PeSeNPs in this work without applying any stabilizer and capping agent. The findings showed that PeSeNPs have a stronger DPPH scavenging activity than other nanoparticles. Under these experimental circumstances, the PeSeNPs sample demonstrated antioxidant activity with an IC50 of 109.11 ± 3.62 µg/ml. DPPH scavenging activity of chitosan selenium (CS-SeNPs), carboxymethyl chitosan-selenium (CCS-SeNPs), and gum Arabic selenium nanocomposites (GA-SeNPs) of 0.6 mM reaches around 80%, according to several types of literature69,70. However, in this study, without the application of any stabilizers or capping agents, the DPPH radical scavenging activity of ASeNPs reached > 80% at 1ml ASeNPs. The findings showed that ASeNPs have a stronger DPPH scavenging activity than other nanoparticles.
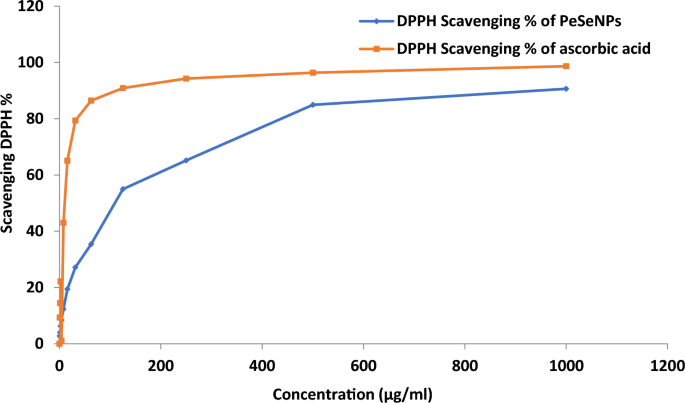
DPPH scavenging activity of PeSeNPs.
Antimicrobial activity of myco-synthesized PeSeNPs
PeSeNPs didn’t have any remarkable antifungal potential on Penicillium expansum RCMB 001001 (1) IMI 28169 and Penicillium marneffeii (RCMB 001022). But a strong promising inhibitory action was recorded against Penicillium italicum RCMB 001018 (1) IMI 193019 and represented with diameters: 16 ± 0.5 mm, which was near to that done by ketoconazole antifungal antibiotic on the same genus; 18.2 ± 0.41 mm. However, a promising antibacterial effect was exerted by our myco-fabricated NPs on Methicillin-Resistant Staphylococcus aureus (MRSA) ATCC 4330 and Porphyromonas gingivalis RCMB 022001 (1) EMCC 1699; 11.9 ± 0.6 mm and 15.9 ± 0.6 mm, respectively. It was noticed that this inhibitory action exerted due to PeSeNPs was very near to that was done by the referenced antibiotics on the examined microbial pathogens. However, the 2 studied bacterial infections can be controlled in terms of growth by gentamicin; MRSA ATCC 4330, and Porphyromonas gingivalis RCMB 022001 (1) EMCC 1699, by inhibition clear zone; 15.0 ± 0.5 mm and 18.1 ± 0.5 mm, respectively, also, ketoconazole antibiotic can inhibit the growth of tested fungal pathogens; Penicillium expansum RCMB 001001 (1) IMI 28169, Penicillium italicum RCMB 001018 (1) IMI 193019 and Penicillium marneffeii (RCMB 001022) with obvious inhibition zones; 17.1 ± 0.5 mm, 18.2 ± 0.4 mm and 12.9 ± 0.6 mm, respectively, as shown in Table 1. Results were done in triplicate and mentioned as mean ± standard deviation. This was supported by the observations of Vahidi et al.7 who stated that the SeNPs myco-fabricated by P. chrysogenum PTCC 5031; exhibited antibacterial efficacy with zones of inhibition (ZOI) of 10 and 13 mm, respectively, towards gram-positive bacterial pathogens such Staphylococcus aureus and Listeria monocytogenes. In the same line with our results, the study done by Rudrappa, et al.40 mentioned that the silver nanoparticles prepared from Plumeria alba leaf (P-AgNPs) showed an antimicrobial potential depending on concentration, in which, higher inhibition was observed at 100 µg/mL P-AgNPs. The strongest antimicrobial potential was recorded towards (S. pneumoniae and E. faecalis) and C. glabrata fungus, on the other hand, the minimum antimicrobial potential was measured towards (E. coli and E. aerogenes). Another study done by Math, et al.47, mentioned that a high potential of antimicrobial was exerted by bio-fabricated silver nanoparticles using extract of Cardamine hirsuta (L.) leaf was noticed in P. aeruginosa by zone of inhibition reached approximately 16.8 ± 0.2, 20.5 ± 0.5, 22.3 ± 0.5 and 23.4 ± 1.0 mm at different concentrations prepared from the NPs prepared from the extract (25, 50, 75, and 100 µL). The lowest action measured on S. aureus with 11.5 ± 0.3, 13.3 ± 0.3, 14.6 ± 0.5, and 16.3 ± 1.0 mm zones of inhibition at various NPs volumes of (25, 50, 75, and 100 µL), respectively. A finding stated by Abbas et al.,71 mentioned that SeNPs with particle size ranging from 32.08 to 103.82 nm as showed by TEM micrographs fabricated from by Fusarium semitectum, could have promising antimicrobial potential against numerous bacterial pathogens such as S. aureus, P. aeruginosa, A. baumanni, and K. pneumonia.
Minimum inhibitory concentration of PeSeNPs
Minimum Inhibitory Concentration (MIC) of PeSeNPs on microbial-examined strains was detected. Promising inhibition was observed in the PeSeNPs concentration of 500μg/ml against Penicillium italicum RCMB 001018 (1) IMI 193019 and Methicillin-Resistant Staphylococcus aureus (MRSA) ATCC 4330; however, the growth was significantly inhibited at 1000μg/ml of PeSeNPs against Porphyromonas gingivalis RCMB 022001 (1) EMCC 1699 as illustrated in Table 2. The mycosynthesized PeSeNPs had superior antifungal activity against Penicillium italicum RCMB 001018 (1) IMI 193019 compared to other tested fungal strains. In accordance with our research, Gunti et al.72. manufactured SeNPs by an aqueous extract of Emblica officinalis fruit and recorded that SeNPs had more promising antibacterial activity towards the tested Gram-positive bacterial pathogens (S. aureus MTCC 96, Enterococcus faecalis MTCC 439 and Listeria monocytogenes MTCC 657) than other examined Gram-negative (E. coli MTCC 4), with (MIC) recorded of about 9.16 µg/mL, 16.17 µg/mL, 33.17 µg/mL and 59.83 µg/mL towards S. aureus, E. faecalis, L. monocytogenes, and E. coli, respectively. For example, Tran et al. 73. mentioned the preparation of Se-NPs stabilized by polyvinyl alcohol and observed the action of SeNPs that significantly inhibits growth of S. aureus at a concentration as low as 1µg/mL, however at all of the tested concentrations, no growth inhibition of E. coli was seen.
Transmission electron microscope
The TEM data offer a more comprehensive understanding of the cellular morphological deformations (Fig. 5A,B) showing the TEM micrographs of Penicillium italicum RCMB 001018 (1) IMI 193019 treated with PeSeNPs. The thin cross-section of individual Penicillium italicum RCMB 001018 (1) IMI 193019 cells is regarded as a control sample visible in micrographs Fig. 5A; as only cylindrical-shaped hyphae with rigid and obvious internal organelles. Once Penicillium italicum RCMB 001018 (1) IMI 193019 was treated with myco-synthesized PeSe-NPs; Fig. 5B, abnormal hyphal morphology with detached membrane and loosen appearance for the internal organelles observed, resulted in unusual impairment of hyphal membrane integrity. The fungal hyphal morphology showed a dramatic change from normal penicillium hyphae to a distorted structure with a broken weakened membrane.
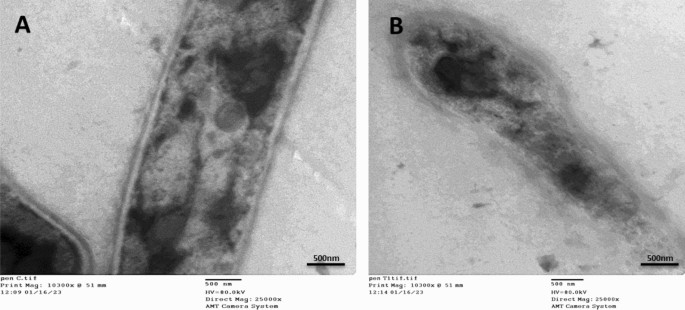
Transmission Electron microscopy images showing morphological alterations in Penicillium italicum RCMB 001,018 (1) IMI 193,019 hypha treated by myco-synthesized PeSeNPs. (A) showed the untreated Penicillium italicum RCMB 001,018 (1) IMI 193,019 exhibit a compact cell wall, continuous cytoplasmic membrane, homogeneous and electron-dense cytoplasm. (B) by contrast, the effect of treating P. italicum species with our myco- synthesized PeSeNPs; an obvious destruction and detachment of membrane and internal organelles integrity, subsequently, changes in hyphal shape formation of pores and cell death.
Tran et al. and Zonaro et al.73,74; the following: size of the particle, shape, surface charge, surface chemistry, and hydrophilicity are significant factors that affect how microbial cell membranes are disrupted. SeNPs’ potent electrostatic repulsion with bacterial membrane charge may be the cause of their greater antibacterial effectiveness towards gram-positive bacteria than gram-negative bacteria71. Tran et al.72, illustrated that Gram-positive bacteria have a significantly lower membrane-negative surface charge than Gram-negative bacteria, which makes it easier for Se-NPs to deposit on their surfaces and induce bacterial harm. As a result, Se-NPs are typically resistant to gram-negative bacteria. Guisbiers et al.75 noted that chemisorption is a potential method for accessing the Se-NPs in the bacterial cell. Lipopolysaccarides are found in the gram-negative bacteria’s outer membrane, which Braun’s lipoprotein uses to form a covalent bond with the peptidoglycan of the cell. Gram-positive bacteria’s cell wall, which has a thicker peptidoglycan membrane, lacks the outer lipopolysaccharide membrane. Therefore, it would seem that SeNPs enter gram-positive bacteria through chemisorption considerably more readily. The selenium NPs disrupt both the wall and the membrane of the bacterial cell, penetrate the cell, frequently overproduce reactive oxygen species (ROS), interfere with ATP synthesis and respiratory sequence, cause protein denaturation, inhibit the activity of enzymes, cause DNA damage, and other effects that collectively cause the internal metabolism to fail consistently, leading to cell death73.
Anticancer activity
In vitro studies
PeSeNPs cytotoxic activity
Ehrlich ascites carcinoma EAC Cells were handled with various concentrations of Se-NPs 5, 10, 20, 40, 80, 125, 250, 400, and 500 µg/ml, and the cytotoxicity (Table 3, Fig. 6) transpired in ways necessary for its anticancer efficacy. All concentrations showed cytotoxic activity except (5, 10, 20, and 40 µg/ml), which showed 0% inhibition and 100% cell viability.
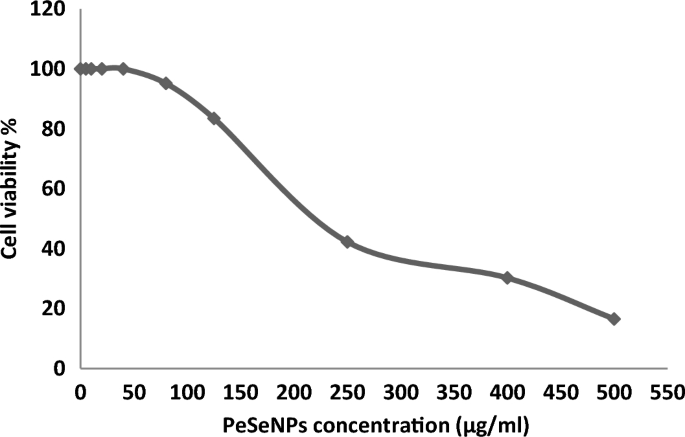
Effect of different doses of PeSeNPs on EAC cell viability assessed using the MTT assay, which indicates the cytotoxic activity against EAC cells with 50% cell cytotoxic concentration (IC50 = 225 ± 0.56 µg/ml).
Kong et al.76 concluded that PeSeNPS inhibited Ehrlich ascites carcinoma (EAC) cell growth partially through caspases-mediated apoptosis and inhibited the transcriptional potential of the androgen receptor by downregulating its mRNA and expressing its protein. Apoptosis is a key mechanism for suppressing cancer growth, and caspases in turn cause apoptosis, as shown in (Fig. 8) by the difference between (Fig. 7A) of control cells without treatment at 100% and cell viability and (Fig. 7B) of cells treated with the maximum concentration of PeSeNPS (225 µg/ml) and cell viability 16.54%. Furthermore, selenium nanoparticles regulate Mdm2 degradation via the pathway of EAC tumor cell growth suppression by the breakage of androgen receptor, and phosphorylation promoting Akt-dependent androgen receptor, as well as increasing Akt kinase phosphorylation in order to be activated, act as a promising.
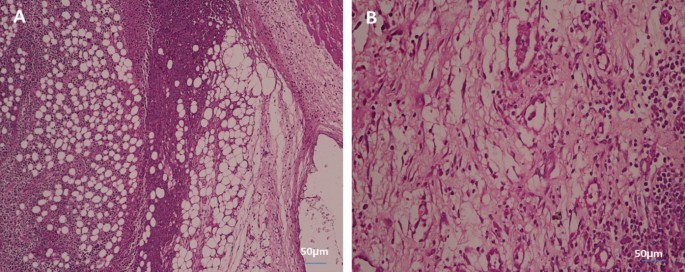
Cytotoxicity of PeSeNPS toward EAC. (A) The control EAC without PeSeNPS, and (B) EAC at a 225 µg/mL concentration.
In vivo studies
Tumor volume
At the day 14, we start determining any change in tumor volume (Fig. 8). Tumor growth was consistent in control (EAC), with infrared radiation only, with PeSeNPs only, and by combining infrared radiation with PeSeNP groups. The relative tumor volumes (V/Vo) on the 14th day are 13.4 ± 0.32 in EAC group, 10.1 ± 0.35 in the infrared radiation group, and 8.2 ± 0.41 in the PeSeNPs group. Our findings showed that both infrared irradiation treatment alone and PeSeNPs injection alone didn’t exhibit a remarkable reduction the tumor development, while in the case of combination therapy (PeSeNPs + infrared radiation), there is a maximum remarkable decrease in tumor volume reached 6.2 ± 0.11, which indicates shrinkage and disappearance of tumor. Infrared light plus PeSeNPs provide powerful photothermal medication and hold promise for other biomedical uses.
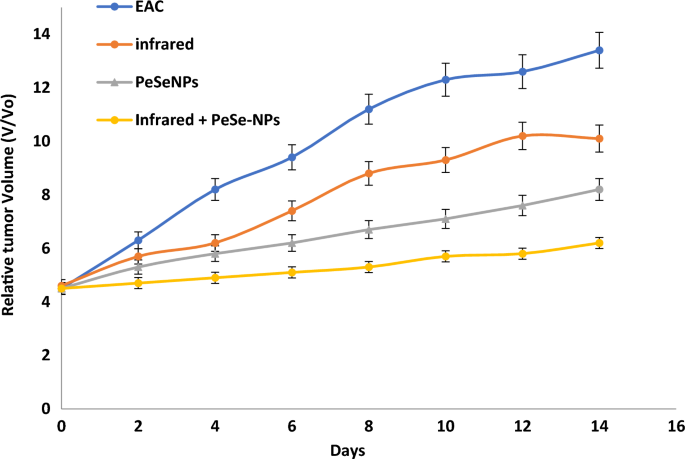
Effect of different treatments showing change in the relative tumor volume.
Mortality rate and survival curve
The rate of mortality is shown in Table 4. There is no percentage mortality of mice that received PeSeNPs and then were irradiated with infrared light. This indicates that the mice’s biological system is improving. Combined therapy has been observed for inhibiting the growth of tumor cells. Figure 9 Show the survival curve of mice after numerous treatments.
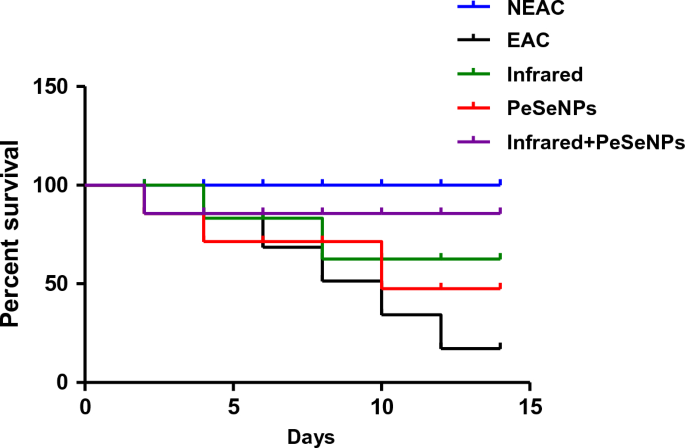
Survival rate of the mice after different treatment.
Liver enzymes and kidney functions
Table 5. shows an increase in AST and ALT liver enzymes as well as kidney functions, urea, and creatinine in tumor-bearing mice based on the formation of tumor cell growth, which leads to an increase in liver enzymes and kidney functions and therefore there is toxicity in liver and kidney tissue as a result of the tumor development, resulting from excessive stress, which causes interactive oxidative damage, which in turn leads to dysfunction in the liver and kidneys. The observations showed that the group handled with infrared ray and selenium nanoparticles had an improvement in kidney enzymes and kidney functions, which was more effective than infrared ray treatment alone or Nano alone because the mixed treatment had excellent bioavailability due to its low toxicity, strong absorption capacity, and catalytic efficiency. It improves liver and kidney activity and reduces tumor cell metastasis and apoptosis as Se-NPs can modify many genes related to the cell cycle and apoptosis that consequently prevents cancer.
Histopathological examination
In tumor tissue
Figure 10 shows the histology of cancer cells stained with hematoxylin and eosin (H&E) (Scale bar: 50 µm). Figure 10A shows tumors without treatment that have inflammatory cells (green arrow). The tumor was irradiated with infrared light only (white arrow, Fig. 10B) and the tumor was also injected with PeSeNPs (orange arrow, Fig. 10C). The two groups showed the beginning of tumor cells shrinkage, but tumors injected with PeSeNPs and then irradiated with infrared light completed necrosis of malignant cells in the core area (Blue arrow, Fig. 10D), therefore PeSeNPs and infrared irradiation illustrated maximum effective destruction of EAC tumor tissue in the comparison to the group of the control77,78.
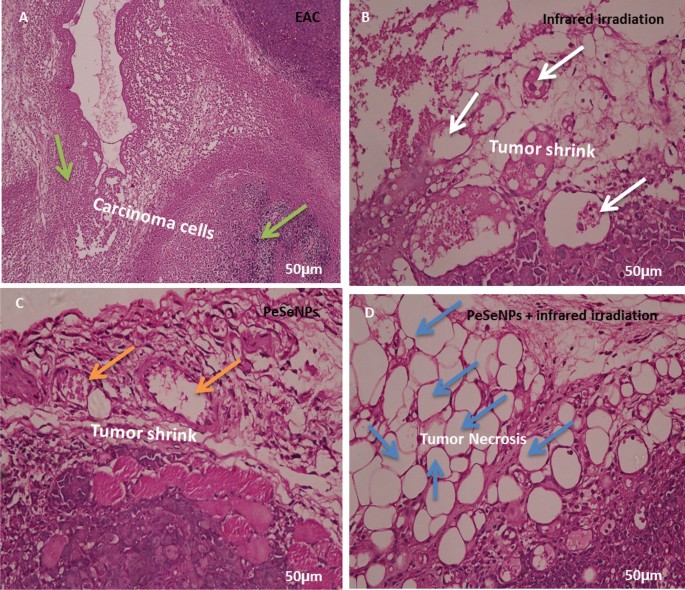
Histological analysis of tumor stained with hematoxylin and eosin (Scale bare: 50 µm). (A) Non treated tumor which shows inflammatory cells (green arrow) (B) Tumor irradiated with infrared which shows shrink of carcinoma cells (white circle) (C) injected tumor with Se-NPs which shows the beginning of cell necrosis (orange arrow circle) (D) Tumor injected with Se-NPs then exposed to infrared radiation which showing necrosis cells (blue arrow).
In liver tissue
Figure 11 shows the histology of liver cells stained with hematoxylin and eosin (H&E). Figure 11A indicates the histology of the liver section of the negative control group, which shows a normal architecture of the liver, central vein (CV), and blood sinusoids (BS). The liver section of the group with untreated tumors shows degenerated hepatocytes with congested central vein (CV), and leucocytes infiltration (LI) (Fig. 11B). The liver section of the group irradiated the tumor with infrared which showed leucocytes infiltration (LI) (Fig. 11C). The liver section of the group injected the tumor with SeNPs which showed bile ductules (BD) and vacuolated hepatocytes with pyknotic (P) nuclei congested central vein (CV) (Fig. 11D). The liver section of the group injected tumor with SeNPs then irradiated by infrared light showing normal architecture of the liver, central vein (CV), and blood sinusoids (BS) (Fig. 11E).
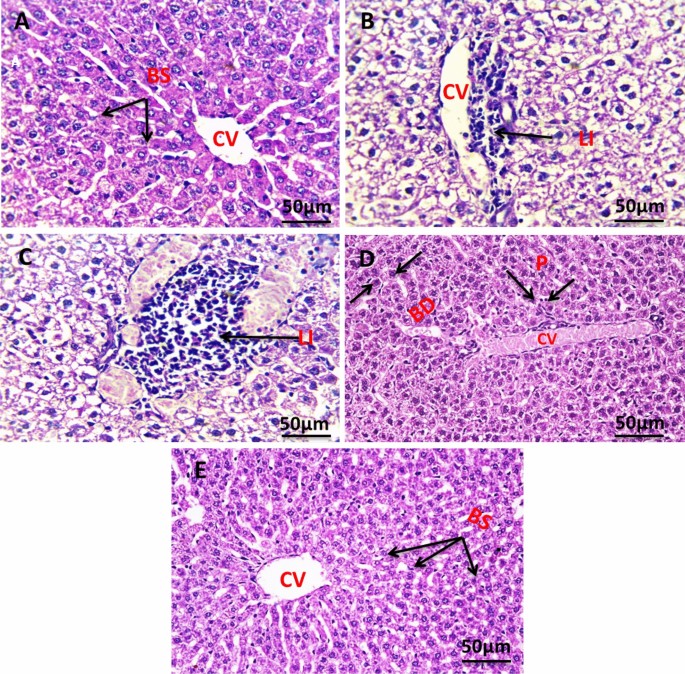
Photomicrograph of liver section of negative control group NEAC showing normal architecture of the liver, central vein (CV), and blood sinusoids (BS) (H. & E., 400X) (A). The liver section of the group with untreated tumor showed degenerated hepatocytes with congested central vein (CV), leucocytes infiltration (LI) (H. & E., 400X) (B). Liver section of group irradiated tumor with infrared which shows leucocytes infiltration (LI) (H. & E., 400X) (C). Liver section of group injected tumor with SeNPs which showed bile ductules (BD) and vacuolated hepatocytes with pyknotic (P) nuclei congested central vein (CV) (H. & E., 400X) (D). The liver section of the group injected tumor with SeNPs then irradiated by infrared light showing normal architecture of the liver, central vein (CV), and blood sinusoids (BS) (H. & E., 400X) (E).
In kidney tissue
Figure 12A revealed the histology of the renal section of the negative control group showing normal renal structure of the glomeruli (G), and renal tubules. The kidney section of the group with an untreated tumor, which shows a cross-section in the cortex and medulla region of the kidney in the exposed group, shows degenerative glomeruli with massive aggregation of abnormal nuclei around blood vessels with hemorrhage (Fig. 12B). The kidney section of the group was irradiated with infrared, which showed massive congestion of blood vessels with hemorrhage and vacuolar degeneration in renal tubules with an abnormal nucleus (Fig. 12C). The kidney section of the group injected the tumor with PeSeNPs which showed marked glomerulus sclerosis (GS), pycnotic nuclei (PN), vascular dilation (VD), and necrosis (Fig. 12D). The kidney section of the group injected the tumor with SeNPs then irradiated by infrared light showing normal glomeruli (G), distal tubules (dt), and Bowman’s space (Fig. 12E).
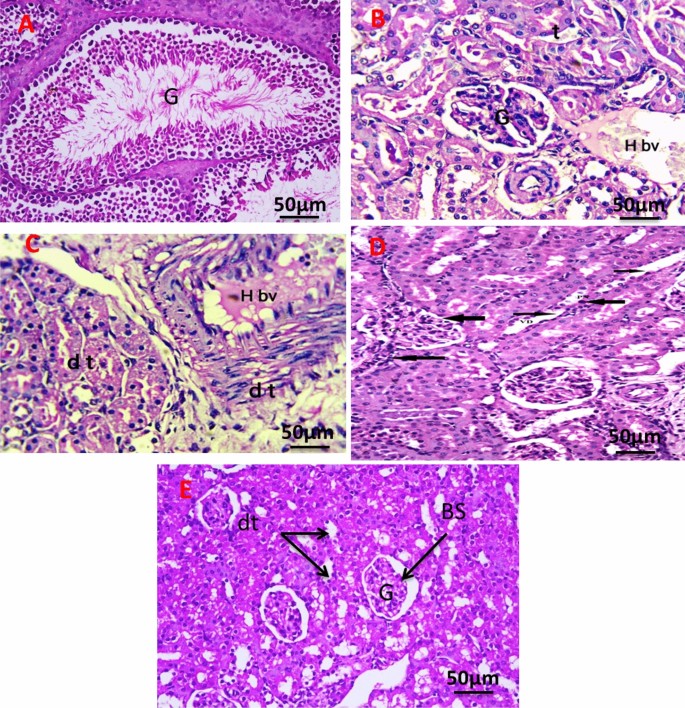
Photomicrograph of Kidney section of negative control group (A). The kidney section of group with untreated tumor (B), irradiated tumor with infrared (C), injected tumor with Se-NPs (D), and injected the tumor with Se-NPs then irradiated by infrared light (E). All slides were stain by (H & E., 400X).
Liver transmission electron microscopy
Figure 13 shows TEM photomicrographs of liver tissue for all experimental groups. The negative control group (Fig. 13A) showed that the cytoplasm contained a round pigmented nucleus (N) having rough endoplasmic reticulum (RER), oval mitochondria (M), and a large nucleolus (Nu). EAC group (Fig. 13B) demonstrated that the nuclei of hepatocytes had clumped, thick chromatin that was arranged erratically into nucleoli (N), and slightly swollen mitochondria (M) and RER dilatation. Infrared group (Fig. 13C) showing Hepatocyte with pyknotic (P) nucleus Illustrating parts of two hepatocytes (H1) and (H2) attached to each other, dilated bile canaliculi (BC) with fragmented microvilli, nucleus (N), rough endoplasmic reticulum (RER), and polymorphic mitochondria (M). PeSeNPs group (Fig. 13D) displays densely granulated, polymorphic mitochondria (M) with aggregated PeSeNP deposition, as well as irregularly shaped nuclei (N). The combined therapy group PeSeNPs + infrared (Fig. 13E) showed almost normal hepatocytes structure. The cytoplasm contains a round nucleus (N) with a noticeable nucleolus (Nu), mitochondria (M), and (RER) which remain normal.
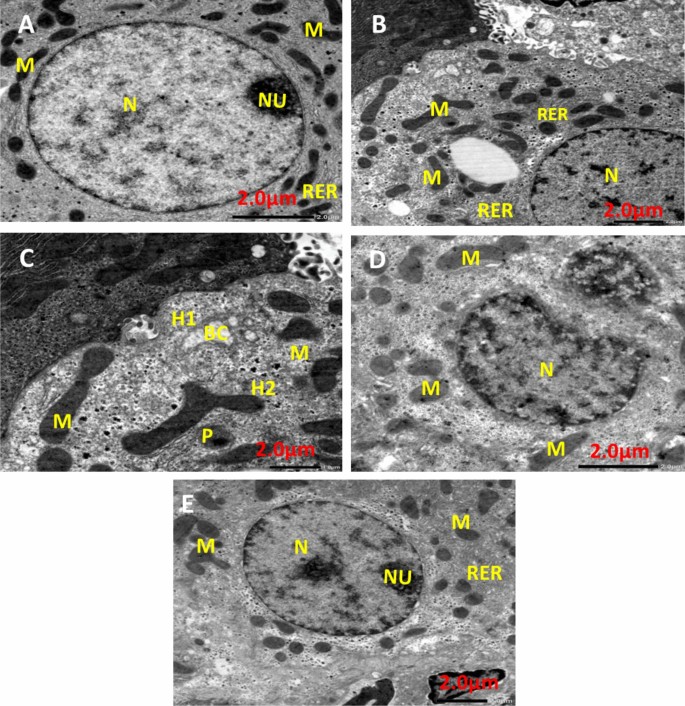
Transmission electron microscope photomicrographs of liver tissue from NEAC, EAC mice treated with, infrared radiation, PeSe-NPs, and PeSe-NPs + infrared radiation.


